Carbohydrates
Carbohydrates are carbon compounds that contain large quantities of hydroxyl groups. The simplest carbohydrates also contain either an aldehyde or a ketone . All carbohydrates can be classified as either monosaccharides, oligosaccharides or polysaccharides. Anywhere from two to ten monosaccharide units, linked by glycosidic bonds, make up an oligosaccharide. Polysaccharides are much larger, containing hundreds of monosaccharide units. The presence of the hydroxyl groups allows carbohydrates to interact with the aqueous environment and to participate in hydrogen bonding, both within and between chains. Derivatives of the carbohydrates can contain nitrogens, phosphates and sulfur compounds. Carbohydrates also can combine with lipid to form glycolipids or with protein to form glycoproteins.
Monosaccharides
The monosaccharides are classified according to the number of carbons they contain in their backbone structures. The major monosaccharides contain four to six carbon atoms.
Carbohydrate Classifications
# Carbons Category Name Relevant examples
3 Triose Glyceraldehyde, Dihydroxyacetone
4 Tetrose Erythrose
5 Pentose Ribose, Ribulose, Xylulose
6 Hexose Glucose, Galactose, Mannose, Fructose

7 Heptose Sedoheptulose
9 Nonose Neuraminic acid, also called sialic acid
The aldehyde and ketone moieties of the carbohydrates with five and six carbons will spontaneously react with alcohol groups present in neighboring carbons to produce intramolecular hemiacetals or hemiketals, respectively. This results in the formation of five- or six-membered rings. Because the five-membered ring structure resembles the organic molecule furan, derivatives with this structure are termed furanoses. Those with six-membered rings resemble the organic molecule pyran and are termed pyranoses
Disaccharides
Covalent bonds between the anomeric hydroxyl of a cyclic sugar and the hydroxyl of a second sugar (or another alcohol containing compound) are termed glycosidic bonds, and the resultant molecules are glycosides. The linkage of two monosaccharides to form disaccharides involves a glycosidic bond. Several physiogically important disaccharides are sucrose, lactose and maltose.
Sucrose: prevalent in sugar cane and sugar beets, is composed of glucose and fructose through an α–(1,2)–β-glycosidic bond.
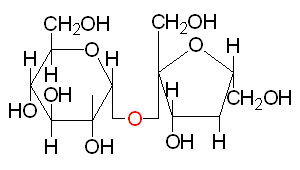
Lactose: is found exclusively in the milk of mammals and consists of galactose and glucose in a β–(1,4) glycosidic bond.
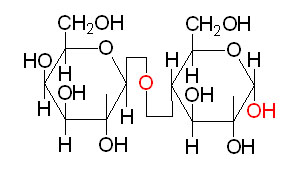
Maltose: the major degradation product of starch, is composed of 2 glucose monomers in an α–(1,4) glycosidic bond.
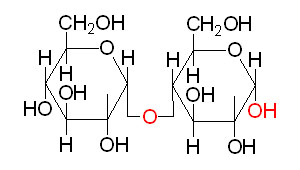
Polysaccharides
Most of the carbohydrates found in nature occur in the form of high molecular weight polymers called polysaccharides. The monomeric building blocks used to generate polysaccharides can be varied; in all cases, however, the predominant monosaccharide found in polysaccharides is D-glucose. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Polysaccharides composed of more than one type of monosaccharide are termed heteropolysaccharides.
Glycogen
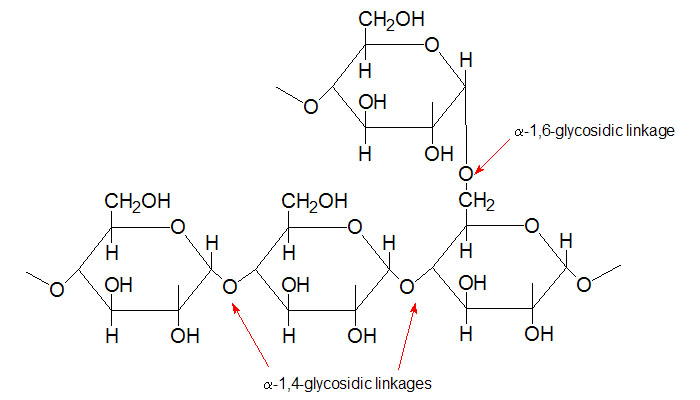
Glycogen is the major form of stored carbohydrate in animals. This crucial molecule is a homopolymer of glucose in α–(1,4) linkage; it is also highly branched, with α–(1,6) branch linkages occurring every 8-10 residues. Glycogen is a very compact structure that results from the coiling of the polymer chains. This compactness allows large amounts of carbon energy to be stored in a small volume, with little effect on cellular osmolarity.
Starch
Starch is the major form of stored carbohydrate in plant cells. Its structure is identical to glycogen, except for a much lower degree of branching (about every 20–30 residues). Unbranched starch is called amylose; branched starch is called amylopectin.
